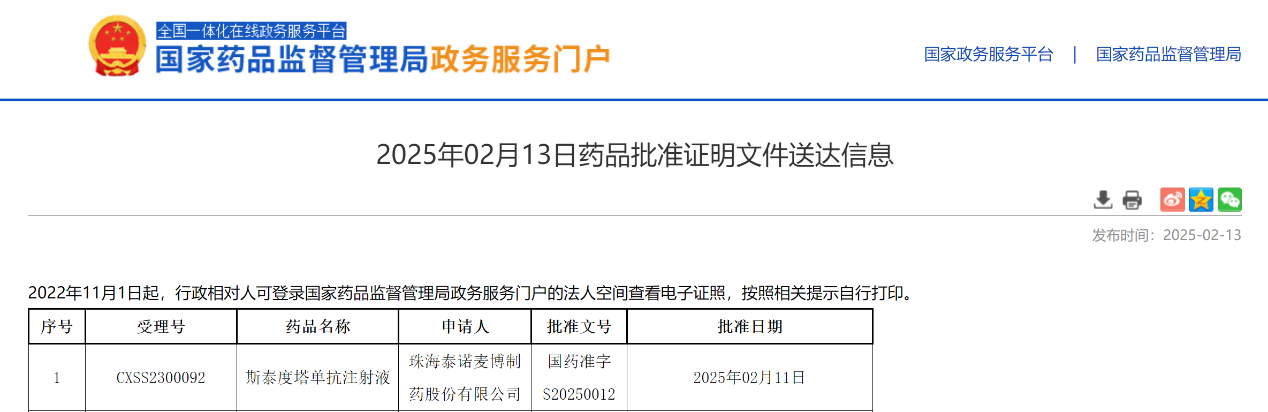
Approval Announcement
On February 11, 2025, Zhuhai Trinomab Pharmaceuticals Co., Ltd. (hereinafter referred to as "Trinomab") announced that its innovative, first-in-class recombinant anti-tetanus toxin monoclonal antibody drug—Sintetol® (generic name: Siltartoxatug Injection,also known as TNM002)—has officially received marketing approval from the National Medical Products Administration (NMPA) of China. This innovative drug represents a significant advancement in tetanus prevention, providing safer and more efficient protection for patients.
Sintetol® is a Category 1 new drug developed by Trinomab. As an upgraded "tetanus shot," it is indicated for emergency tetanus prophylaxis in adults via intramuscular injection. The drug achieves protective levels of anti-tetanus antibodies, outperforming the standard 250 IU HTIG in both efficacy and rapid-onset as well as antibody duration with a single dose, eliminating the need for skin testing, post-dose observation (for outpatient cases), or adjustments based on body weight or wound size.
Threat of Tetanus: A Lethal Risk Demanding Vigilance
Tetanus is an acute, life-threatening disease, with approximately one million global cases reported annually[2]. Without medical intervention, the fatality rate can be as high as 100%, and even with aggressive treatment, mortality remains as high as 30–50%[2]. Clostridium tetani, the causative bacterium, is ubiquitous in soil, dust, and animal feces. Once introduced into the human body through wounds, it can trigger severe infections. Timely and effective prophylaxis is therefore critical.
Limitations of Traditional "Tetanus Shots": Dual Challenges of Safety and Accessibility
Conventional tetanus prophylaxis agents, including Tetanus Antitoxin (TAT) and Human Tetanus Immunoglobulin (HTIG), face significant limitations:
· TAT: Derived from horse serum, TAT carries a high risk of allergic reactions (5–30%), including anaphylactic shock and serum sickness[3]. It has been phased out in many developed countries and removed from the WHO Essential Medicines List since 1991[4].
· HTIG: Dependent on human plasma supply, HTIG suffers from production bottlenecks, frequent shortages, and potential transmission of blood-borne pathogens.
These constraints not only hinder timely patient care but also impose substantial burdens on global public health systems.
Sintetol®: A Revolutionary Advance in Tetanus Prophylaxis
As the world’s first recombinant anti-tetanus toxin monoclonal antibody, Sintetol® has been demonstrated having groundbreaking advantages over current traditional tetanus prevention agents:
1. Safety
o Zero serum sickness reported in clinical trials.
o No skin testing and observation requirements, streamlining administration to a "one-shot, walk-away" protocol.
o Mitigates fatal risks from false-negative skin tests.
2. Efficacy
o Achieves rapid passive immunity with protective antibody levels in 95.4% of patients within 12 hours (vs. 53.2% for HTIG)[1].
o Provides passive immunity with higher protective neutralizing antibody titers and longer duration (up to 105 days) than the standard HTIG.
3. Stability
o Utilizes GMP standardized production facility and gene recombination technology with the international standard quality assurance and quality controls to ensure batch-to-batch quality and consistency of the product.
4. Accessibility
o Production via recombinant gene engineering bypassing reliance on unstable supply of human or equine plasma.
o GMP-certified manufacturing facilities enable industrial scale of stable production, resolving blood supply shortages.
Global Recognition: Dual Endorsements from Chinese and U.S. Regulators
Sintetol® has received regulatory designations in both China and the U.S. In March 2022, China CDE granted Sintetol® Breakthrough Therapy Designation, followed by Fast Track Designation granted by the US FDA in August 2022. Marketing approval in China by China marks a great milestone in tetanus prophylaxis, accelerating global commercial development of antibody therapeutics.

Prof. Chuanlin Wang
Deputy Director, Emergency Surgery/Trauma Center, Peking University People’s Hospital; Lead Investigator of TNM002 Phase III Trial
“Although neonatal tetanus was officially eliminated in China in 2012, non-neonatal tetanus continues to pose a significant public health challenge. Sintetol®, a recombinant monoclonal antibody specifically targeting AB fragment of tetanus toxin, offers a novel approach to enhancing prophylactic strategies against this life-threatening infection”.

Prof. Zijing Liang
Emergency Medicine Specialist, Guangzhou Medical University First Affiliated Hospital
“As an innovative, China-originated recombinant monoclonal antibody against tetanus toxin, Sintetol® has been demonstrated for its promising efficacy in its Phase III clinical trials. The results highlight its rapid onset of action (establishing an immune barrier within 12 hours), superior efficacy (providing sustained high levels of neutralizing antibodies for several months, with titers approximately 5–7 times higher than HTIG), favorable safety profile (eliminating the need for skin testing, with no risk of allergic reactions or bloodborne disease transmission), and enhanced accessibility (high-yield production ensuring stable clinical supply). The approval of Sintetol® is expected to offer an optimized solution for global tetanus prevention and treatment, overcoming the limitations of traditional prophylactic agents”.

Dr. Huaxin Liao
Chairman & CTO, Trinomab
Marketing approval of "Sintetol® was benefited by innovative development of native human monoclonal antibody as therapeutic drug using our proprietary HitmAb® platform. Our enriched pipeline includes also several other monoclonal antibody candidates such as TNM001 targeting respiratory syncytial virus (RSV), which has entered in phase III clinical trials and TNM006 targeting on human cytomegalovirus (HCMV), which has been granted IND approval. Our team has built extensive expertise in development of recombinant human monoclonal antibodies as therapeutic drugs with current focus on infectious diseases, and we will wage our relentless effort to joint world-wide effort in fighting infectious diseases as well as other disorders and promoting public health.

Mr. Weihong Zheng
CEO, Trinomab
"The launch of Sintetol® helps mitigate the reliance on human plasma supply and supports the WHO’s initiative to transition away from animal-derived medical products. This represents a significant step toward safer and more scientifically advanced prophylactic solutions."
Trinomab: Innovating for Global Health
Trinomab remains dedicated to developing clinically impactful therapies. Marketing approval of Sintetol® highlights our commitment to advancing biotechnology and addressing public health needs. We will continue to drive innovation in antibody therapeutics to enhance global health outcomes.
Disclaimer
1. Trinomab does not promote unapproved drugs or off-label use.
2. Medical information herein is for informational purposes only and not intended as healthcare advice.
Prospective Statements
This news announcement contains prospective descriptions subject to risks and uncertainties. Terms like "anticipate," "believe," and "expect" denote such descriptions. Trinomab disclaims any obligation to update these descriptions.
References
[1] Sintetol® Prescribing Information, NMPA Approval Document.
[2] Non-Neonatal Tetanus Diagnosis and Treatment Guidelines (2024 Edition), National Health Commission.
[3] Wang, C. Tetanus. People’s Medical Publishing House, 2022.
[4] MSF Medical Guidelines.