
Platform Technical Features
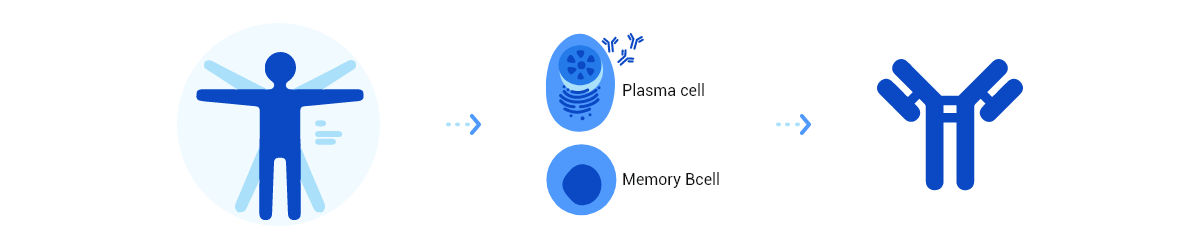
The "High-Throughput Fully Human Monoclonal Antibody Development Integrated Platform HitmAb®" is the company's proprietary core technology for discovering fully human monoclonal antibodies. This platform efficiently screens monoclonal antibodies and identifies the most promising candidates for specific indications. Leveraging HitmAb® as its foundation, Trinomab has developed an exclusive, high-efficiency system for screening and identifying fully human monoclonal antibodies tailored to particular disease treatments.
The platform incorporates advanced technologies, including antigen design, target antibody labeling, flow cytometry sorting, gene amplification and sequencing, and cell transfection. Operating in a high-throughput mode, HitmAb® rapidly isolates single B cells or plasma cells to screen large populations of human monoclonal antibody pools. It then extracts the specific antibody expression genes, which are subsequently cloned and expressed as recombinant therapeutic antibodies for large-scale production in a CHO system.
These recombinant fully human monoclonal antibodies exhibit high adaptability in the human body, along with advantages such as low immunogenicity, high specificity, high affinity, and superior safety. The HitmAb® platform overcomes the time-consuming, labor-intensive, and complex challenges of traditional antibody development methods (e.g., vector cloning), enabling high-throughput and efficient antibody discovery.
Monoclonal antibodies derived from the HitmAb® platform are fully human, having been presented and matured in a human immune tolerance environment. As a result, they minimize endogenous immunogenicity issues common in non-human antibody drugs and reduce anti-drug antibody (ADA) responses during clinical applications. Compared to antibodies from phage libraries or transgenic animal technologies, HitmAb®-derived antibodies undoubtedly offer excellent safety and therapeutic potential for patients.
 Platform Technical Features
Platform Technical Features
 Platform Technical Features
Platform Technical Features
 Platform Technical Features
Platform Technical Features

 Platform Technical Features
Platform Technical Features